sbr
Created by Nancy Desai

Application
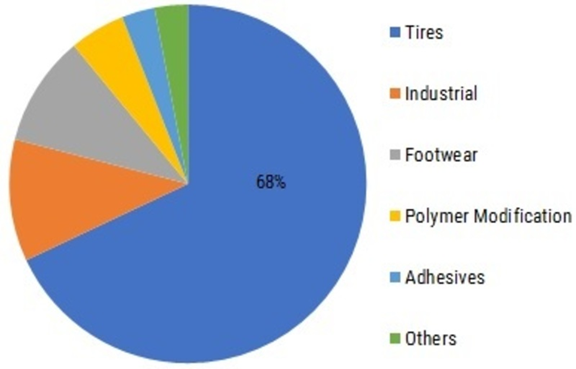
•It is used in car tires and even in heavy-duty . This is because of its high heat resistance.
•In lighter-duty tires, cold emulsion SBR is used.
•In speciality applications, solution SBR is commonly used. This happens because of its high cost—for example, motorcycle treads and radial car tires.
•It is also used in automotive parts . For example ,drive couplings .
•It is used in wire insulation and cabling, belting, roll coverings, haul-off pads, hoses, seals, gaskets, abrasion resistance and metal adhesion.
•In commercial aspects, SBR polymer uses range from shoe soles, moulded rubber goods to carpet backing adhesive.
•SBR is also used as a binder in lithium ion battery electrodes.
Properties
•SBR offers high tensile strength, abrasion resistance and resilience.
•It has decent resistance and is flexible in low temperatures.
•It is also resistant to organic acid, water, chemical, alcohol, ketone, and aldehydes.
•It is crack resistant which allows it to adjust fillers in large amounts hence enhancing its properties.
•Carbon black: Increases the strength, abrasion and UV resistance of SBR.
•China clay: It is used in the production of rubbers that are not black, which adds strength and reinforcement.
•Silica: This increases the thermal conductivity, dimensional stability and electrical insulation.
•SBR also swells and weakens from hydrocarbon oils.
example of SBR



what is SBR?
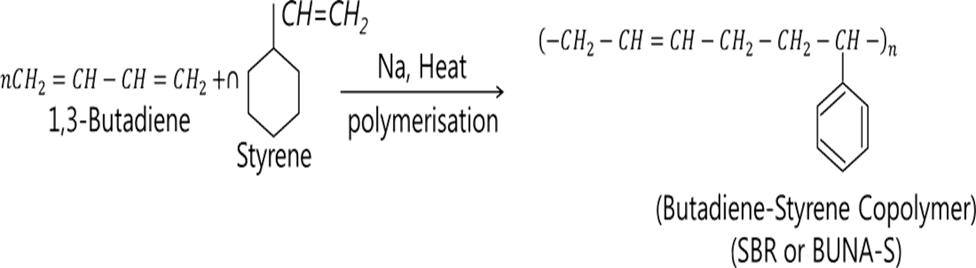
•Styrene-Butadiene Rubber is produced by the free radical polymerization of styrene and butadiene.
•It is a random polymer so it has irregular structure and it is not crystallizable.
•SBR is a polymer with a styrene content in the range of 10 to 25% and butadiene content in range of 60 to 70%.
•The addition of styrene lowers the price and contributes to the good wear and bonding properties.
STRENE BUTADINE RUBBER
stucture
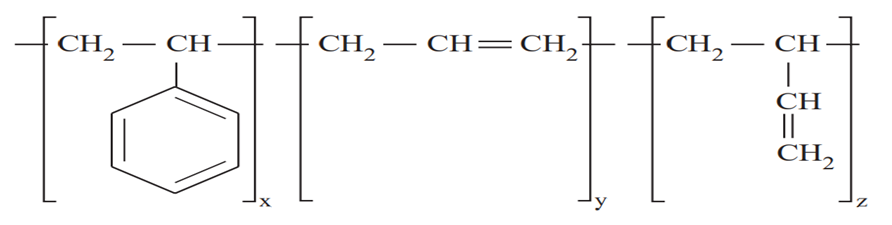
synthesis
There are two types of SBR : (I) Emulsion SBR
(II) Solution SBR
Emulsion SBR
* E-SBR is produced by emulsion polymerization is initiated by free radicals.
(I) Hot E-SBR: It can be produced by free-radical emulsion polymerization of styrene and butadiene at 50 to 60°C.
(II) Cold E-SBR: It can be produced by free-radical emulsion polymerization of styrene and butadiene at about 5°C.
Solution SBR
* S-SBR is produced by an anionic polymerization process.
- Solution SBR is produced by , anionic solution polymerization of styrene and butadiene with alkyl lithium initiator (e.g., butyllithium) in a hydrocarbon solvent, usually hexane or cyclohexane.
synthesis of BuNa-S
•It is a random co-polymer formed by the emulsion polymerization of a mixture of 1:3 butadiene and styrene in the presence of peroxide catalyst at 5 °C and the obtained product is called Styrene butadiene rubber (SBR).
•In Buna-S, Bu stands for butadiene and, Na for sodium and S for styrene. It is vulcanized with sulfur.