Created by Tomas Kulhanek
pharmacolibrary
Tomáš Kulhánek123, Filip Ježek24, Jiří Kofránek23,
Marek Mateják25, Stef Romes1
1Flemish Institute of Technology and Research (VITO), Mol, BE
21st Faculty of Medicine, Charles University, Prague, CZ
3Creative Connections s.r.o, Prague, CZ
4University of Michigan, Ann Arbor, USA
5Institute of Clinical end Experimental Medicine, Prague, CZ
Mol, Belgium
Environmental unit
Digital Precision Health group
Pharmacokinetics (PK) body → drug
Pharmacodynamics (PD) drug → body
Pharmacogenomics (PGx) genetics→drug&body
Administration(absorption), Distribution, Metabolism, Elimination(excretion)
parenteral (e.g. intravenous, main parameters: adminMass,adminDuration):
enteral (e.g. oral, main parameters: adminMass, Tlag, F absorption fraciton, ka absorption rate)
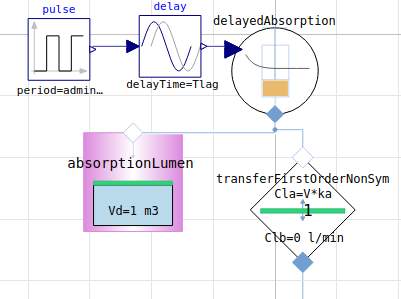
simplified - ideal mixing, no volume flow rate, compartment (Vd volume of distribution)
and 1st order kinetics
compartment - with concentration mixing based on volumetric flow through organs and tissues:
fixed volumetric flow component:
simplified - using 1st order rate
e.g. paracetamol
acetaminophen → sulfate_conjugate
for more complex - use Chemical library
via kidney or liver using clearance rate Cl
parameters for intravenous administration of gentamicin, type of antibiotic against bacterial infections
parameters for intravenous administration of midazolam, a short-acting benzodiazepine used for sedation, anesthesia, ...
compartment takes physiological features into account
1 cup = 100 mg of caffeine in 8 hours
What effect has a drug to body
midazolam
PV shift from right to left → less maximal volume, slight increase in systolic pressure.
Digoxin is a cardiac glycoside derived from the foxglove plant Digitalis lanata. It is primarily used in the treatment of various heart conditions, notably atrial fibrillation, atrial flutter, and sometimes heart failure that cannot be controlled by other medications. Digoxin is approved and widely used in clinical practice today.
Howto systematically approach PGx in Modelica and Pharmacolibrary?
*1, *1
808T, *1
normal
intermediate
*1, *1
*2, *1
*17, *1
normal
poor
rapid
gentamicin
eGolem powered by bodylight.js
https://egolem.online/irm/ https://bodylight.physiome.cz
Pharmacolibrary: https://github.com/creative-connections/Pharmacolibrary
pharmacokinetic: 5496 drugs models - most generated
pharmacodynamics: various effects
pharmacogenomics: various genotype/phenotype influence
current and future research and development: contribute to democratize pharmacology knowledge, use PBPK for quantitative in vitro to in vivo extrapolation (QIVIVE), validate PK models of drugs, add PK of dietary supplements and toxicokinetics of chemicals, cover published PD and common pharmacogenes,
https://github.com/creative-connections/Pharmacolibrary
ONCOSCREEN project, funded by the European Union’s Horizon Europe grant No. 101097036.
subaward to R01 HL173346. funded by NIH
Commercial tools:

, …
Open-source:
i
Frank-Starling law is much more visible in component model - "force of the heart muscle depends on pressure of incoming blood".
A C Guyton, T G Coleman, H J Granger, Circulation: overall regulation, Annu Rev Physiol.1972;34:13-46.doi: 10.1146/annurev.ph.34.030172.000305.
J.Kofránek, et al., Restoration of Guyton‘s Diagram for Regulation of the Circulation ...Physiol Res. 2010;59(6):897-908.doi: 10.33549/physiolres.931838.
connectors and components:
40-50 pharmacogenes commercially available in testing panels
majority cytochrome P450 enzymes ( CYP2D6,CYP2C19,...)